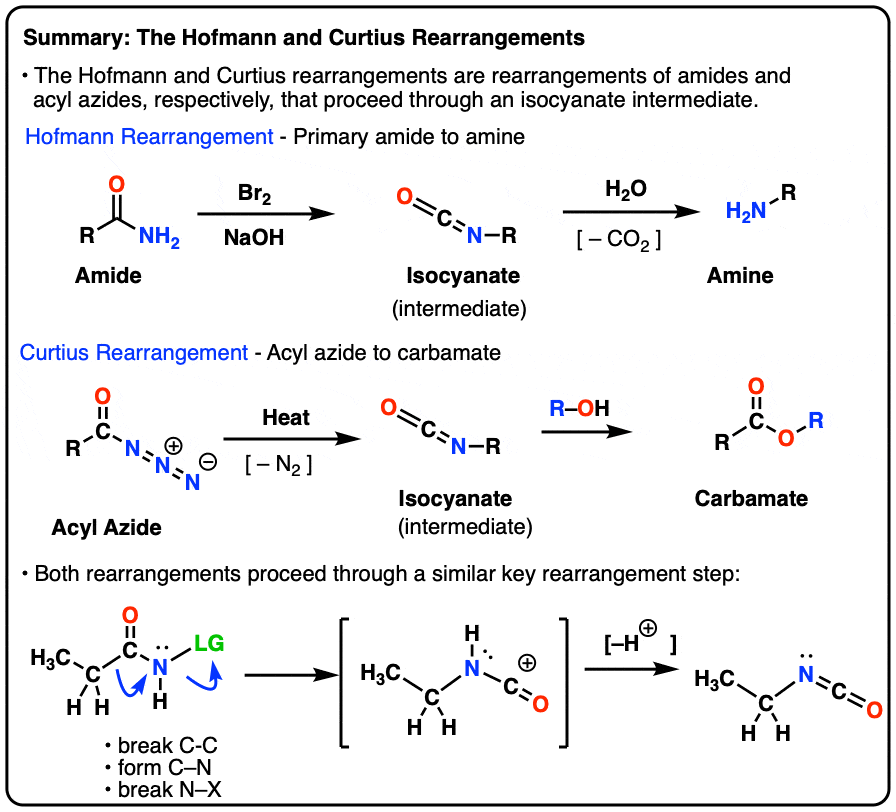
Mechanism for the carbaryl hydrolysis and 1-naphthol radical scavenging... | Download Scientific Diagram

The identification of carbon dioxide mediated protein post-translational modifications | Nature Communications

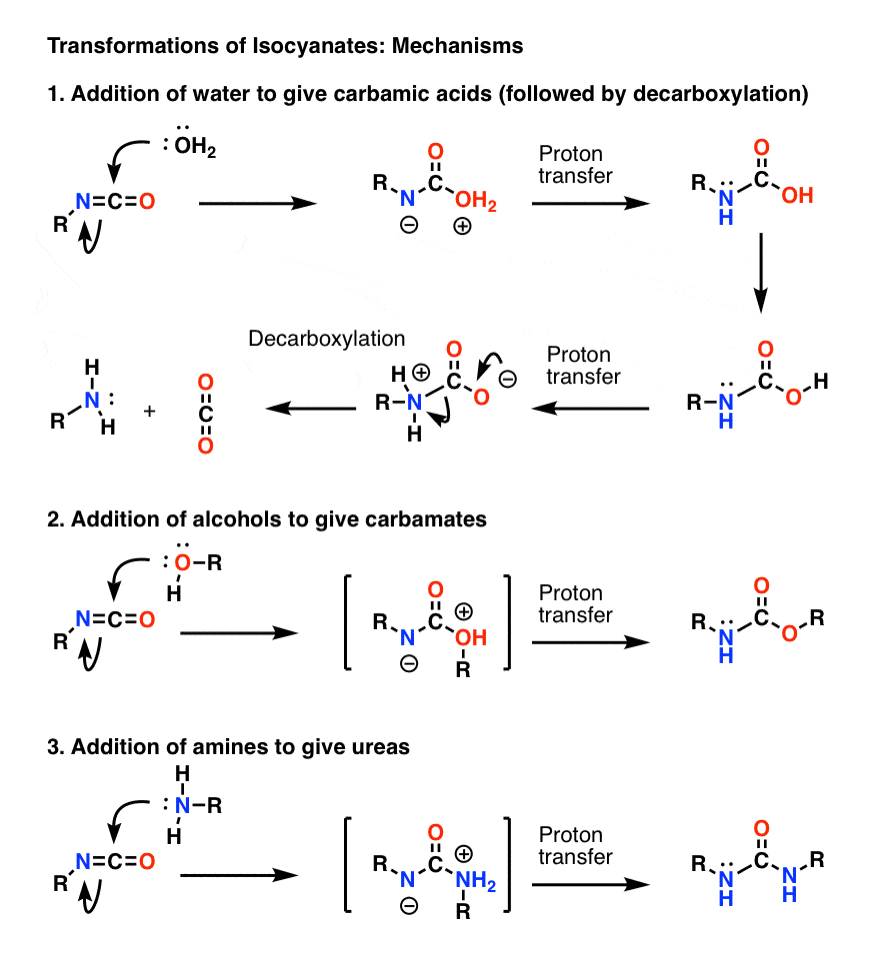
IJMS | Free Full-Text | Hydrolysis Mechanism of Carbamate Methomyl by a Novel Esterase PestE: A QM/MM Approach

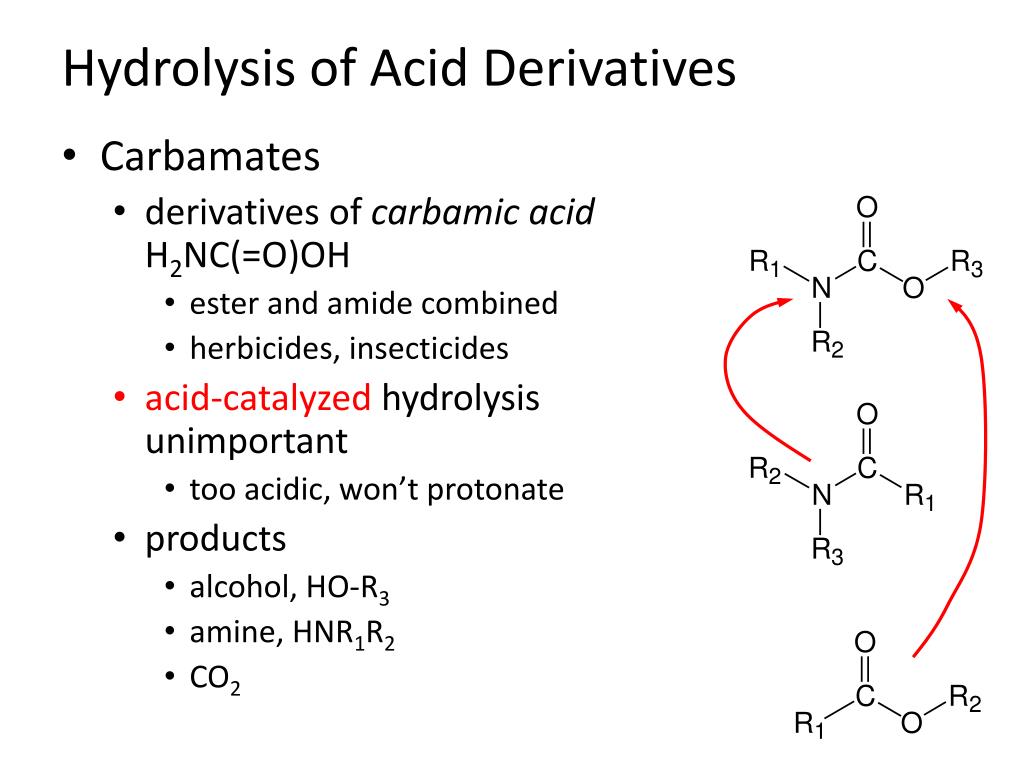
SOLVED: Question 3 (25 points): The carbamate containing compound (3) , releases the free amine rapidly under basic conditions: In contrast; a carbamate is extremely stable to base hydrolysis Describe an arrow

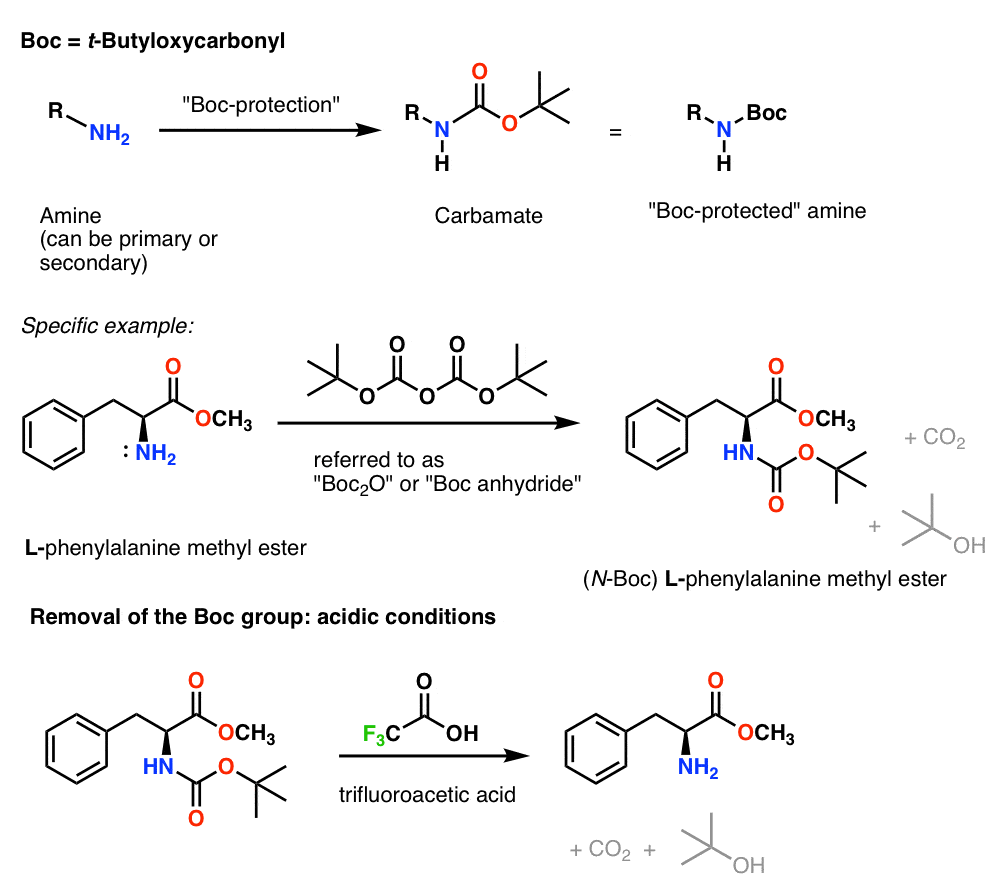
Alkaline hydrolysis of tertiary N-(2-pyridyl)carbamates. Contradictory evidence between nucleophilic and general base catalysis | SpringerLink

Hydrolysis susceptibility and carbamate formation for a low moisture-absorbing, siloxane-modified cyanate ester resin matrix (TC410) material used for composite space applications - Rafael J Zaldivar, Geena L Ferrelli, Hyun I Kim, 2022

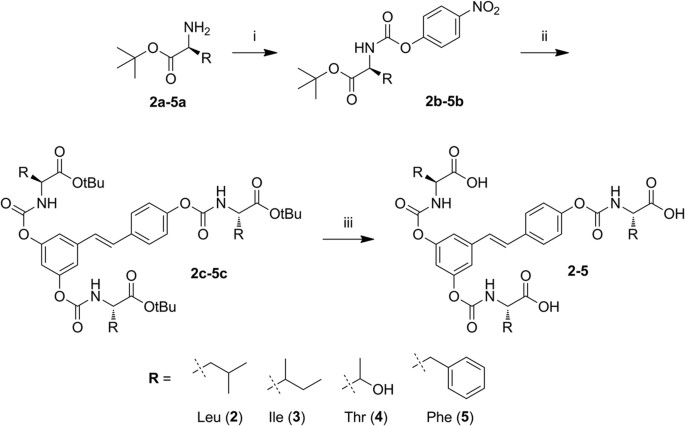
Triazene drug metabolites. Part 17: synthesis and plasma hydrolysis of acyloxymethyl carbamate derivatives of antitumour triazenes - ScienceDirect

SciELO - Brasil - Kinetics and mechanism of hydrolysis of benzimidazolylcarbamates Kinetics and mechanism of hydrolysis of benzimidazolylcarbamates
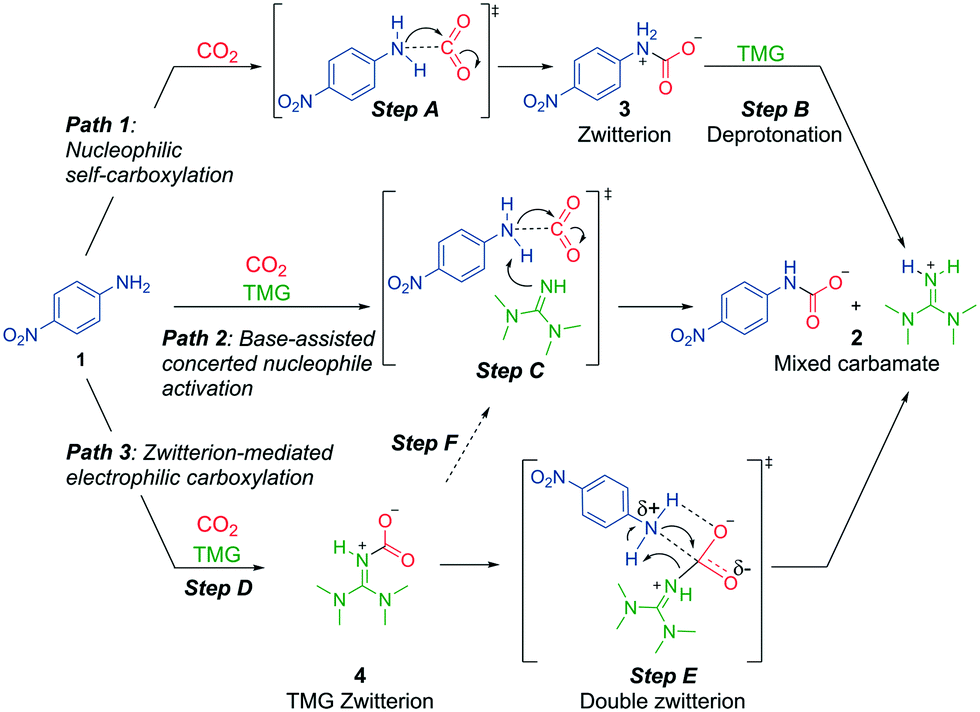
Mechanistic insights into carbamate formation from CO 2 and amines: the role of guanidine–CO 2 adducts - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/D1CY01433A